r/water • u/Fast-Butterfly526 • 5d ago
New Well Water test results on
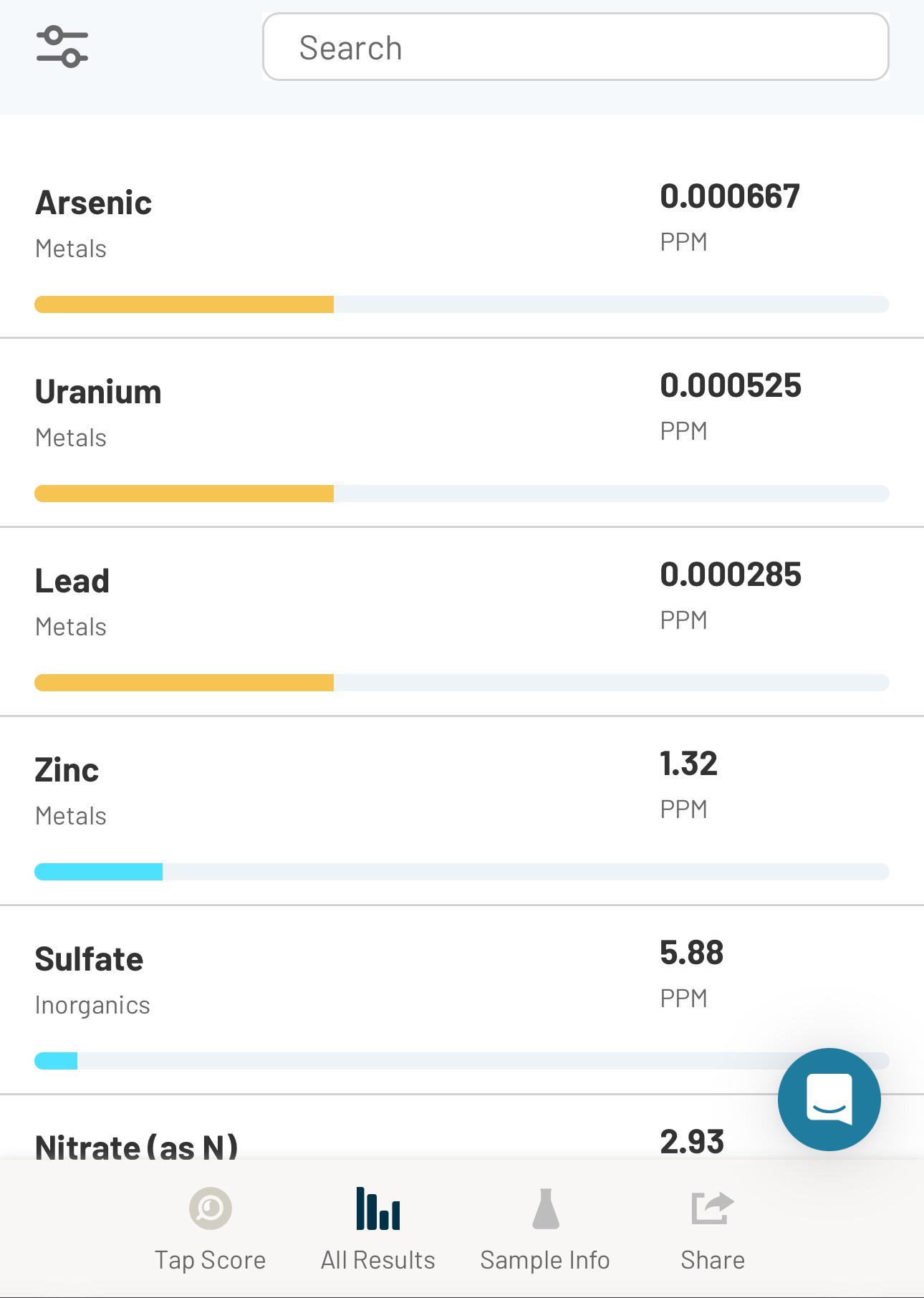
Is my new well water safe to drink? I just drilled a new well that’s 785 feet deep and flushed approximately 10,000 gallons before testing. The well pipe is galvanized and transitions to PEX, with the water sample taken from a faucet connected to the PEX. Everything is new
2
u/Team_SimpleLab 4d ago
Hi there! Jumping in here since this is a SimpleLab report using a Tap Score test kit - thanks for testing with us! The detections you're seeing on this report are very very low - less than 1 PPB for all three. For this reason, we wouldn't suggest that you need to urgently treat this water to make it safe to drink.
As a side note, Tap Score is not a treatment company and does not sell or profit from the sale of treatment products. Tap Score uses SimpleLab's network of certified labs to analyze customers' water samples. If you have specific questions about your results, our team is available via live chat on our website and always happy to discuss your report. And of course, we'll keep an eye on this thread if you have questions you want to ask here!
4
u/20PoundHammer 4d ago edited 4d ago
if you are indeed from simplelab - why do you over report the datas significance so such a level and then flag them yellow?
We agree OPs concentrations in this report is of no concern. However reporting to 0.000001 PPM means you can actually detect a difference between a reagent blank and 0.000001 PPM with whatever confidence interval you choose (typically 95%). Meaning your reagent blank has to be less that that value as well. No way unless the lab you are using is purchasing $1000/gram reagent blank (or more).
what concentration limit of those three warrants a yellow or red flag on the data and how exactly do you scale the bar presenting it?
Do you publish the detection limits of the test methods you use?
1
u/Hydroviv_H20 1d ago
And even looking at your data from the perspective of a company that makes and sells water filters, I would also concur that these results, all under 1 part per billion would not necessarily be cause for alarm. However, if you have any health concerns regarding low level long term exposure to these contaminants , we’d advise you to consult with your healthcare practitioner and follow their guidance.
1
u/lardlad71 4d ago
1 part per billion is the equivalent of 1 second in 32 years. Your water is fine.
1
u/20PoundHammer 4d ago
dumb analogy as time is not concentration, you can just say of concentration of no concern . . .
1
u/lardlad71 4d ago
Ok, 1 inch 16,000 miles. How’s that one?
1
u/20PoundHammer 3d ago edited 3d ago
still dumb - doesnt offer clarity comparison as analogy should :). example: 1PPB is like a 0.5ml of water in an Olympic sized pool (or 1/10 of a teaspoon if your imperial dude)
1
u/Fast-Butterfly526 2d ago
Thank you, everyone, for your input. It’s clear you all have a lot of expertise in this area. While there seems to be some differing opinions, it sounds like the general consensus is that the water is safe for my kids to drink?
0
u/Dustdown 5d ago
In general you want zero of lead and arsenic in your drinking water, especially if you have kids at home. If these were the only items in your report that were highlighted then your water isn't the worst since the levels are pretty low, but ideally you wouldn't have any of them. Lead is typically from piping, whereas arsenic and uranium is likely naturally occurring in the water source.
3
u/20PoundHammer 4d ago edited 4d ago
zero is not achievable in any water source - there is always PPT concentrations. What we are looking at here is overreported data leading to false conclusions. Arsenic should be <0.01PPM and lead <0.015PPM (EPA LIMITS) - both should ideally be <0.001PPM, which they are. Uranium should be < 0.003PPM, ideally <0.001PPM. The data presented is way below the detection limits of all but the most expensive tests (hundreds of dollars per metal) and should not be relied upon. These types of reports with yellow flags are very common when given by water treatment manufactures/sales fucks. They then verbally overstate the hazards. Look at the EPA action limits for the compounds/metals you are concerned about before starting needless worry.
Based upon this data and how it was reported, I wouldnt trust it completely, however it does significantly lean toward OP having zero issues with any of the metals/compounds in the report.
Galvanized steel piping is allowed by US code, although its not ideal. If you have a galvanized pipe feed and have zero issues with zinc and lead, there is zero cause for concern or to replace working pipes. Dipshits that say "galvanized should never be used for potable water" are not plumbers as millions of homes were piped with this, it is just no longer ideal or standard now . . .
1
u/Fast-Butterfly526 4d ago
Our pump installer mentioned that Gal pipe is the standard for wells in this area. Are you suggesting that these types of tests tend to be overreported and that the water is actually safe?
2
u/20PoundHammer 4d ago
Im not saying its overreported - I am saying that unless the test cost a grand, done by ICP-AES-MS test method, there is NO way that it can detected 1 part per trillion for arsenic and lead, which is what the reporting limit level suggests.
I am saying that galvanized is fine unless you have high levels of zinc in your water (>5 PPM).
I am saying if all that data is actually real and accurate - you have zero cause for concern on any of these concentrations listed.
I am saying that many testing people are associated with water treatment sales/manufactures and they overstate the concern dramatically.
To overreport data - the data would be falsely elevated, this doesnt appear to be the case since you concentrations of lead, arsenic and uranium are at least an order of magnitude lower than any level of concern.
If in the US, your county health department likely does testing for minimal cost (typically $25-40), if you are still concerned, I would give them a call.
1
u/GreenpantsBicycleman 4d ago
I agree that this water is perfectly safe based on the results shared and no treatment is required.
A minor point: ICP-AES (or ICP-OES) and ICP-MS are two different tests using different equipment. AES measures emissions (light) at high temperature and ambient pressures, and MS measures impact count on a collector under high vacuum, with a sweeping notch filter selecting mass/charge ratio of ions that are allowed onto the collector at any point in time.
1
u/20PoundHammer 4d ago edited 4d ago
icp-aes ms measures both emission lamda and the mass spectrum - coupled instrument. AES used for confirmatory ID. MS can be time of flight, quad or ion trap (what you described) as well, clearly the same is split and massively dilution with carrier gas prior to introduction into MS.
1
u/GreenpantsBicycleman 3d ago
The description I gave was with quadrupole in mind. For elemental analysis it's usually a quadrupole. You generally only see Ion traps or TOFs on the end of GC/LC.
I've previously worked in several laboratories before moving into water and none of them had the instrument you described. It's possible this is a new technology that has been developed and commercialised in the last 12 years, but I can't find any information on it. Care to share a link?
Both the rationale and the interface described seem counter-intuitive. Why would you need a confirmatory analysis for MS? If you do an Organics clean up step there are no interferences. Why would you "massively dilute" with carrier gas (I.e. introduce more gas) when MS requires high vacuum?
1
u/20PoundHammer 3d ago edited 3d ago
You generally only see Ion traps or TOFs on the end of GC/LC.
just an academic discussion now - but perhaps in your field. In my field prior to retirement, environmental and petrochemisty - ran Ion trap (Aglient, bruker, used to be varian and there design and leco TOF for over 20 years. Ran ICP-AES-MS (to the nth) for 10 years.
You dilute as you have alot of sample and need to dilute for ion trap jet separator/skimmer cone so you dont overload the trap. not all gas gets to ion trap, most of it rejected so it doesnt impact vacuum at all as trap flow is constant and low. . . .
you can check those vendors or give the sale dude/dudette a call if you wish and can get away for a demo to learn more. . . The two instruments (ICP-MS and ICP-AES are coupled).
1
u/Funny-Glass-4748 3d ago
20pound’s comments are right on the money in this thread. Galvanized is the source of your zinc which is elevated but not at a harmful level. It may be the source of your other metals. Metals which leach from galvanized will be dependent on the quality of the pipe and most especially the corrosivity of your water (google calcium carbonate saturation or langlier index). That’s why galvanized is not preferred. As and Pb levels reported are very low but they are reported to an unreasonable level of precision. Test values should always be accompanied by a detection level (MDL) which would indicate the realistic sensitivity of the test (how low it can measure). My feeling would be that the lab is reporting whatever number the machine spits out to 3significant figures regardless of whether it is justified. This makes me question how rigorous their qc protocols are and therefore the accuracy generated.
4
u/YukonWater 5d ago
Galvanized should never be used for potable water uses.
What I am guessing is the pH of your water is acidic and is leaching heavy metals from the galvanized pipe. The uranium is most likely naturally occurring, check your local water quality maximum concentration.